Dermatology
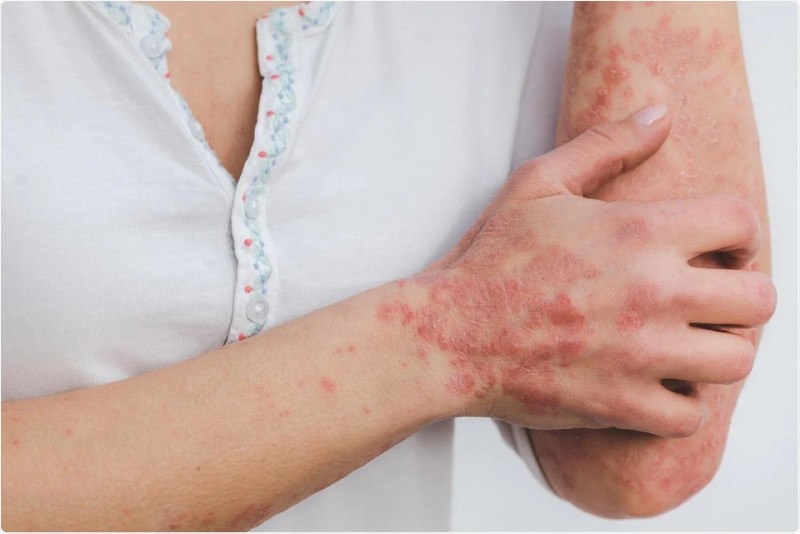
Guselkumab is Safe for Long-Term Psoriasis Treatment, Does Not Raise Cancer Risk
-
 Diabetology1 week ago
Diabetology1 week agoDiabetes Screening: Why Early Detection Saves Lives
-

 Diabetology7 days ago
Diabetology7 days agoGlycemic Index Chart + Tips: Best & Worst Foods for Blood Sugar
-

 Diabetology1 week ago
Diabetology1 week agoCaring for Diabetes Daily: A Practical Guide to Routine Control
-

 Diabetology5 days ago
Diabetology5 days agoDaily Diabetes Management Plan for Type 2 Diabetes
-
 Diabetology1 week ago
Diabetology1 week agoHyperglycemia: What You Need to Know to Stay Safe
-

 Diabetology5 days ago
Diabetology5 days agoUnderstanding the Impact of High Glucose Levels
-

 Diabetology3 days ago
Diabetology3 days agoHow Real-Time Blood Glucose Monitors Improve Health Outcomes
-

 Diabetology3 days ago
Diabetology3 days agoHow a Plant-Based Diet Can Help Manage Type 2 Diabetes