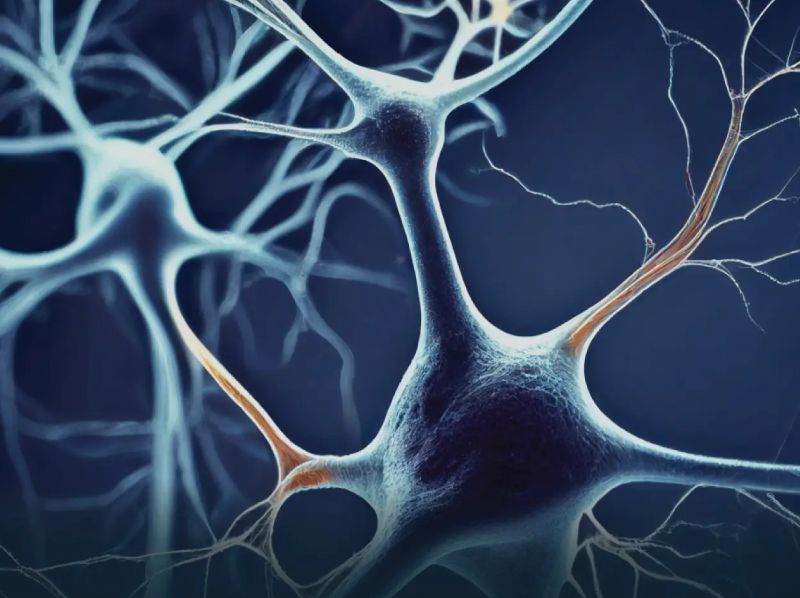
Findings from a study that was recently published in Neurology Neuroimmunology Neuroinflammation showed that aquaporin-4 immunoglobulin seropositive (AQP4-IgG+) neuromyelitis optica spectrum disease (NMOSD) may have cytomegalovirus (CMV) infection as a possible cause.1 These findings point to the possibility of NMOSD T-cell receptors (TCRs) as indicators of the effectiveness of immunosuppression in illness patients.
The complementarity determining region 3 (CDR3) of the TCR repertoire was substantially less diverse and shorter in the 302 patients with AQP4-IgG+ NMOSD (n = 151) compared to the healthy controls (n = 151). Additionally, 597 NMOSD-TCRs with a high degree of sequence similarity were found in the investigation, and these TCRs may be useful in the diagnosis and prognosis of NMOSD patients.
“The characterization of NMOSD-TCRs and pathology-associated clonotype annotation indicated that the occurrence of AQP4-IgG+ NMOSD may be associated with CMV infection,” senior author Hongyu Zhou, PhD, professor of neurology at West China Hospital, Sichuan University, Chengdu, China, and colleagues wrote. “This was further corroborated by transcriptome and single-cell B-cell receptor (BCR) analysis results from public databases and T-
Between October 2014 and March 2017, high throughput TCR sequencing was carried out on the peripheral blood of healthy individuals and pretreated patients with AQP4-IgG+ NMOSD recruited from West China Hospital. After that, the TCR repertoire of people with NMOSD and healthy people was compared, and the results showed that TCR clones were significantly more abundant in people with NMOSD.
Moreover, patients with AQP4-IgG+ NMOSD (n = 28) treated with immunosuppressants were followed up for a very long time to look at the progressions in NMOSD-explicit TCRs pre-and posttreatment. To further investigate the causes of AQP4-IgG+ NMOSD, T-cell activation experiments with CMV antigenic epitopes were carried out, as were transcriptome and single-cell BCR data from public databases.
In contrast to healthy individuals, patients with NMOSD had a higher proportion of nonfunctional TCR clones and a lower diversity of TCRs, according to the findings. Notably, the shorter CDR3 sequences that the researchers found in NMOSD-TCRs suggested that there may be an increased risk of self-recognition, which could result in an autoimmune disease.
Overall, the findings demonstrated that NMOSD may be associated with viral infections on the basis of the clonotype analysis and antigenic epitope predictions. Patients with NMOSD also enriched in viral infection and innate immunity pathways, as shown by the transcriptome data. A specific antibody clonotype known as AQP4-IgG1, which had a higher affinity for AQP4, and elevated interferon-related and viral infection-related gene expression in patients with NMOSD lend credence to the possibility of a connection between NMOSD and CMV infection.
Patients with NMOSD whose TCR characteristics changed after treatment suggested that NMOSD-TCRs may serve as prognosis predictors, particularly for those who responded well to treatment. The authors hypothesized that CMV causes self-reactive T cells to clonally expand, which in turn activates innate immune signaling pathways and causes neural demyelination, which in turn contributes to the pathogenesis of NMOSD. In addition, the crossreactive antigenic epitopes that appear to interact with AQP4 between CMV and C. perfringens may indicate that multiple pathogens are involved in NMOSD due to their distinct genetic backgrounds in distinct populations.
“We performed antigen peptide stimulation experiments in vitro to verify that CMV infection is associated with NMOSD. Initially, these experiments indicated that CMV antigen peptides could activate antigen specific CD4+ T cells from the periphery of patients with NMOSD, which was not found in healthy individuals. According to Zhou et al., “it is suggested that CMV may be involved in the pathogenesis of NMOSD through the activation of self-reactive T cells.”
Regarding the limitations, the authors mentioned that the antigen preference of the database might have an impact on the pathology clonotype annotation of NMOSD-TCRs. They also said that more research is needed to prove that NMOSD could be linked to CMV infection. To determine whether CMV participates in the pathogenesis of NMOSD by activating self-reactive T cells through molecular mimetic mechanisms, the authors recommended additional antigen crossreactivity experiments and validation in animal models.

 Diabetology2 weeks ago
Diabetology2 weeks ago
 Diabetology2 weeks ago
Diabetology2 weeks ago
 General Medicine1 week ago
General Medicine1 week ago
 Diabetology2 weeks ago
Diabetology2 weeks ago
 Diabetology2 weeks ago
Diabetology2 weeks ago
 General Medicine1 week ago
General Medicine1 week ago
 Diabetology4 days ago
Diabetology4 days ago
 Diabetology4 days ago
Diabetology4 days ago